Technology
Technological process
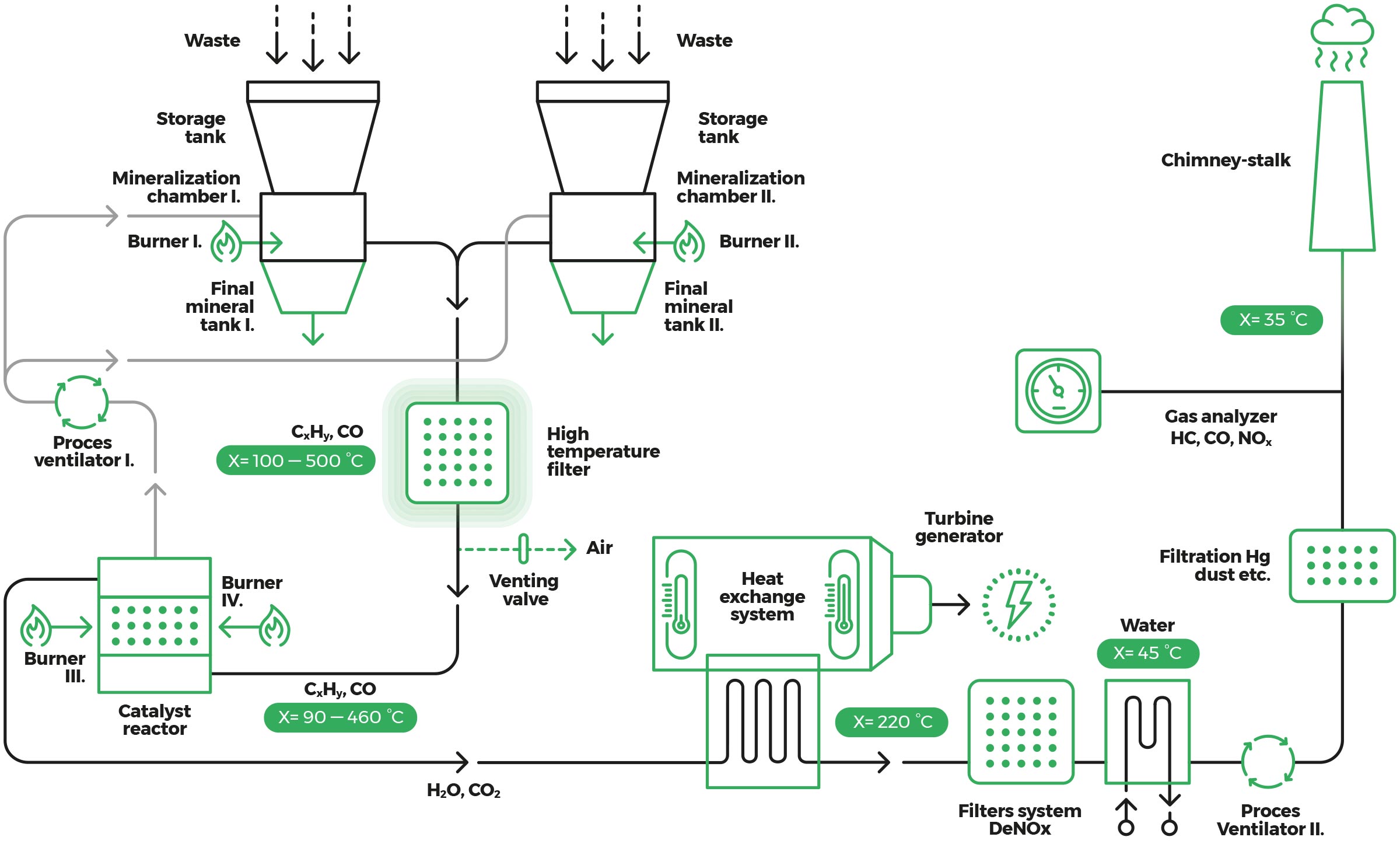
After treatment of the input waste by separation and crushing to the desired fraction up to 50 mm, it is conveyed by the conveyor to the reaction chamber storage chamber from which the reaction chamber is gravitationally filled.
The reaction chamber is heated to the operating temperature after approx. 450 °C. When the chamber is filled with waste, all humidity is evaporated from it to a temperature of 200 °C and the process of mineralization, gasification and ullitisation begins. Temperatures range from 500 to 550 °C depending on the type of waste being treated.
The process of mineralization leads to material drying and subsequent decomposition. Carbon is gasified to CO2 or other organic compounds that are distributed on the catalysts to CO2 and H2O. All carbon is consumed as the ongoing reactions shorten the gas hydrocarbon chains that are catalytically distributed on CO2 and H2O and the inert material depending on what is found in organic matter.
The metal and glass components found in waste in the mineralization process are not oxidized due to the low process temperature and negligible oxygen presence of 3%, and they are unaltered from the process. It can be said that the process that takes place in the reaction chamber is anaerobic.
Catalytic mineralization is a process in which organic materials form a mineral.
Input waste volume is reduced by 20 - 100 times depending on its composition.
Processed waste is discharged from the reaction chamber in the form of a fine mineral powder and is transported from the hopper located at its lower part through the conveying conveyor to a collecting container.
This terminates the process of mineralization and starts the process of technical purification of gases. The temperature is adjusted to 400 °C in order not to damage the catalysts. The reaction gas is transported to high-temperature filters through which it flows and is cleaned from all impurities and minerals so as not to damage the catalysts.
Subsequently, it is transported to a catalytic dopant where it is oxidized by air as a result of which its temperature rises to 600 °C. The reactor runs a reaction that changes CxHy and CO to H2O and CO2. From the reactor, 5% of the hot gas is transported back into the reaction chamber, which heats up and no longer needs to be heated by a gas burner. The remaining 95% of the hot gas goes into a heat exchanger that works on the principle of exchange (air - air, air - water, air - oil). The heat exchanger reduces the temperature from 600 °C to 220 °C. The exchanger can be air or steam to a turbine generator that produces electricity by power or goes into the DeNOx catalytic reactor, where it reduces NOX to NO2.
Catalysts work at optimum temperature. The gas decomposes on the catalysts to produce CO and CO2 and NOx compounds. The chlorine compounds decompose from the gas, which are further processed or alkalized (adsorbed on the medium) on sorbents to produce CO2. The reaction gas is subsequently purified in a reverse oxidizer. The cleaning level is 99.9%.
Subsequently, the gas is transported to a heat exchanger where its temperature drops from 220 °C to 35 °C and from there it is transported into a chimney where it is discharged from the air as a vapor of CO2 and H2O. If we capture H2O, which is in the volume of 600 l / t of waste into the atmosphere, only CO2 is drained.
Scheme
The basic scheme of the municipal waste disposal facility.